Enzyme-enriched condensates show self-propulsion, positioning, and coexistence
Leonardo Demarchi, Andriy Goychuk, Ivan Maryshev, Erwin Frey
What are generic consequences of coupling local enzymatic catalysis to molecular interactions between enzymes and substrates in chemically active biomolecular condensates? To address this question, we studied a minimal model of enzymes that show liquid-liquid phase separation and convert a single substrate species into product, which in turn is eventually restored to substrate.
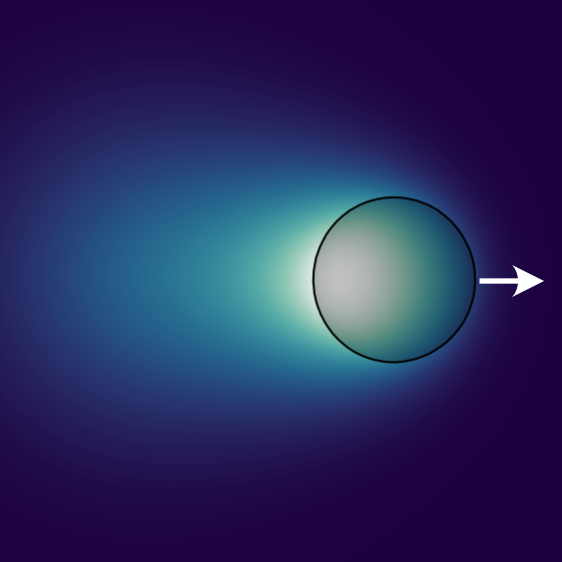 Within this minimal model, the enzymatic droplets can exhibit self-propulsion without relying on surface interactions or hydrodynamic coupling. When individual droplets self-propel by polarising, we observe oscillations in closed domains. Even if the droplets do not self-propel, they can still find the geometric center of the container, with dynamics resembling a damped harmonic oscillator. When multiple droplets are placed in the same container, they can equilibrate their sizes, positioning themselves equidistantly in the container. Finally, in conjunction with the molecular interactions, the reaction kinetics can reverse the coarsening process and induce droplet divisions while conserving the overall number of enzymes. Thus, non-equilibrium reactions can significantly affect the dynamics of condensate formation.
Within this minimal model, the enzymatic droplets can exhibit self-propulsion without relying on surface interactions or hydrodynamic coupling. When individual droplets self-propel by polarising, we observe oscillations in closed domains. Even if the droplets do not self-propel, they can still find the geometric center of the container, with dynamics resembling a damped harmonic oscillator. When multiple droplets are placed in the same container, they can equilibrate their sizes, positioning themselves equidistantly in the container. Finally, in conjunction with the molecular interactions, the reaction kinetics can reverse the coarsening process and induce droplet divisions while conserving the overall number of enzymes. Thus, non-equilibrium reactions can significantly affect the dynamics of condensate formation.
The model's simplicity allows for an analytic treatment and explanation of the various observed phenomena. This study sets an important framework to address the dynamics of active catalytic droplets. It could have significant implications for the biophysics of molecular condensates, including mid-cell localisation in prokaryotic cells. Finally, it highlights the importance of molecular interactions in shaping intracellular chemistry.

