Directing Min protein patterns with advective bulk flow
Sabrina Meindlhumer*, Fridtjof Brauns*, Jernej Rudi Finžgar*, Jacob Kerssemakers, Cees Dekker, and Erwin Frey
We explored the effect of advective bulk flow on the dynamics of membrane-bound protein patterns. We approach this question theoretically and experimentally by studying how Min protein patterns respond to bulk flow. Our key findings include the following:
- Membrane-bound Min protein waves can be directed by external bulk flow.
- Counterintuitively, Min patterns can flow with or against the applied bulk flow, depending on the MinE: MinD concentration ratio.
- Flow can be used as a versatile experimental tool to uncover the distinct molecular mechanisms of pattern formation.
There is evidence that intracellular flows play a significant role in pattern-forming processes, such as in the monocellular stage of the C. elegans zygote. However, there have been few studies focusing on this crucial aspect. This study used the well-established Min protein system as a model for intracellular pattern formation. In previous studies, Min protein waves were found to propagate upstream in the presence of fast bulk flow, with their wavefronts moving against the direction of the flow. The authors proposed that the influence of MinE bulk gradients and the rapid switching of MinE between reactive and latent states caused this phenomenon.
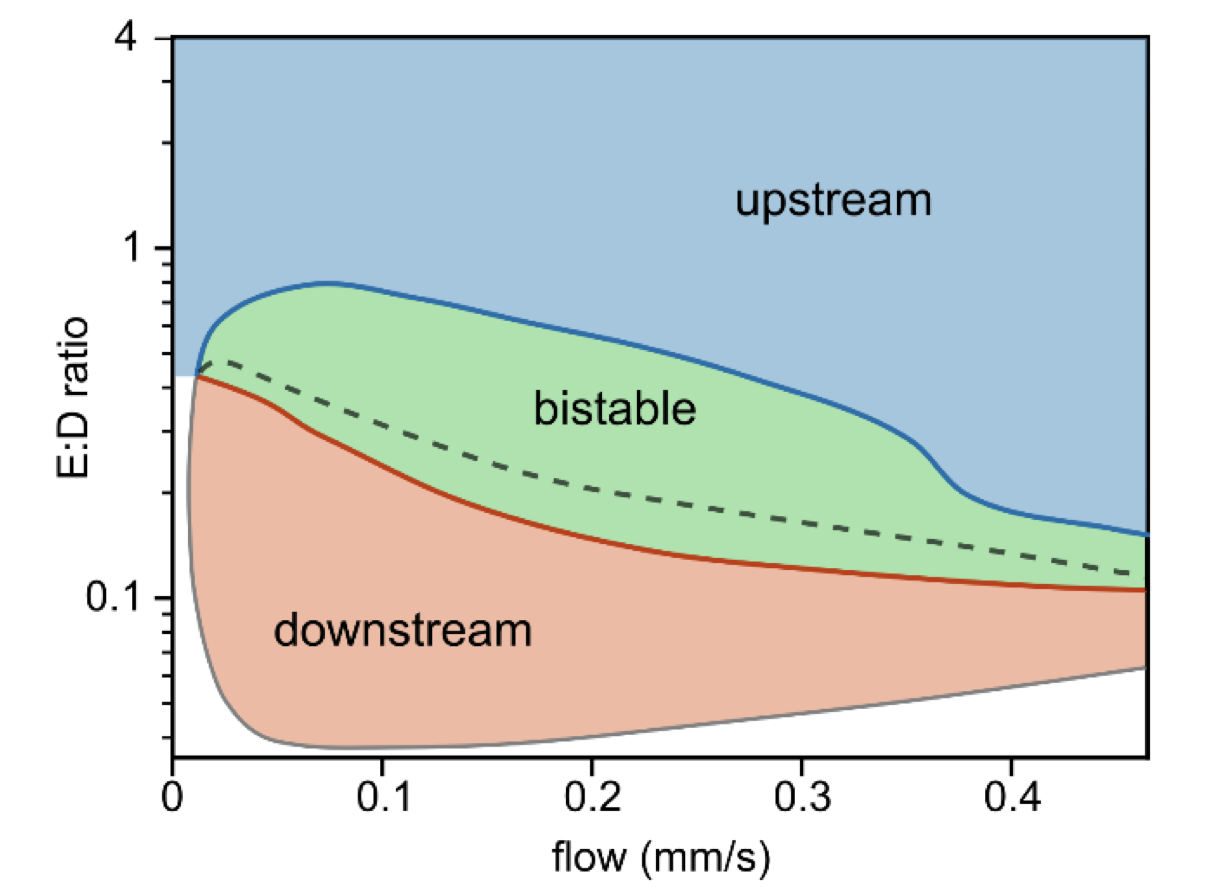 Inspired by this work, we asked whether bulk flow could be used as a probe to reveal the underlying molecular mechanisms of the Min system. Notably, bulk flow acts differently, shifting bulk protein gradients but not directly affecting proteins already bound to the membrane. This suggests that the response to bulk flow is sensitively dependent on the bulk-surface coupling due to the attachment and detachment of proteins at the membrane. Building on this idea and using a theoretical model including the MinE switch, as proposed by Denk et al. (2018), we predict upstream propagation for high MinE: MinD ratios and downstream propagation for low MinE: MinD ratios. We confirmed these predictions experimentally using confocal microscopy data and quantitative analysis of Min patterns. We also predict multistability and hysteresis between high and low MinE: MinD ratios and flow rates in the intermediate regime. Our experimental data confirm these predictions, with the observed patterns' responses to flow showing a strong dependence on their initial state without flow.
Inspired by this work, we asked whether bulk flow could be used as a probe to reveal the underlying molecular mechanisms of the Min system. Notably, bulk flow acts differently, shifting bulk protein gradients but not directly affecting proteins already bound to the membrane. This suggests that the response to bulk flow is sensitively dependent on the bulk-surface coupling due to the attachment and detachment of proteins at the membrane. Building on this idea and using a theoretical model including the MinE switch, as proposed by Denk et al. (2018), we predict upstream propagation for high MinE: MinD ratios and downstream propagation for low MinE: MinD ratios. We confirmed these predictions experimentally using confocal microscopy data and quantitative analysis of Min patterns. We also predict multistability and hysteresis between high and low MinE: MinD ratios and flow rates in the intermediate regime. Our experimental data confirm these predictions, with the observed patterns' responses to flow showing a strong dependence on their initial state without flow.
Additionally, we performed a systematic model reduction demonstrating that MinE bulk gradients do not cause upstream propagation, as previously suggested, but by advective transport of MinD in bulk. Our work sheds light on the critical question of how protein patterns respond to external perturbations. Without spatial cues, Min protein patterns have no intrinsic directional preference. Symmetry breaking occurs spontaneously, as evidenced by the numerous classical Min patterns observed in vivo and in vitro. External bulk flow, however, imposes a preferential direction on the pattern, causing their wavefronts to move along that direction. A critical insight from our analysis is that advective mass flow can be used as a versatile probe to uncover various molecular mechanisms, such as the role of the Min switch in this study, as well as to reveal other hidden molecular features of the Min protein network in future research. At the same time, this setup can also be used as a control parameter for Min protein patterns, which may have interesting applications in areas such as microfluidics.

