How kinesin motors can reverse their direction
Himanshu Pandey*, Emanuel Reithmann*, Alina Goldstein-Levitin, Jawdat Al-Bassam, Erwin Frey†, Larisa Gheber†
In this interdisciplinary work, we have combined experimental and theoretical approaches to reveal the mechanism underlying directionality switching of kinesin motors – a long-standing problem in the field of motor proteins.
The majority of kinesins are plus-end directed and their catalytic domains are N-terminally located. Conversely, members of the minus-end-directed kinesin-14 family carry their catalytic domains at the C-terminus. However, nearly a decade ago, it was discovered that some mitotic kinesin-5 motors (with N-terminal catalytic domains) are minus-end directed and, moreover, can switch directionality under various experimental conditions. These surprising findings challenged the accepted paradigm that kinesin motors are exclusively unidirectional – and refuted the notion that kinesins with catalytic domains at or near the amino-terminus are plus-end directed. In spite of numerous subsequent experimental and theoretical studies, the mechanisms underlying the fascinating directionality switching of kinesin motors remain unknown.
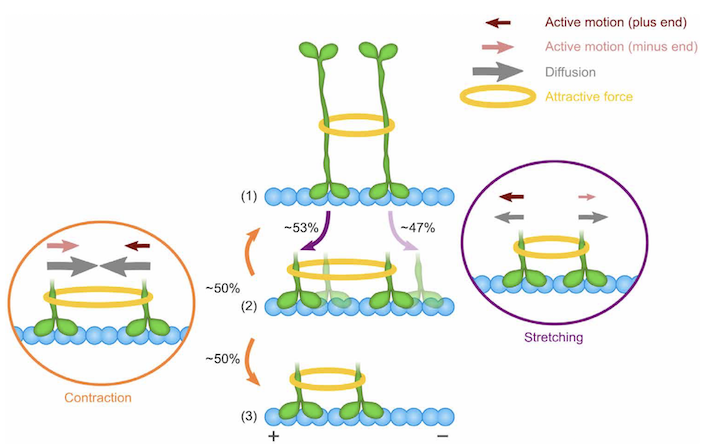
We studied clustering-induced directionality switching of the mitotic kinesin-5 Cin8. To this end, we used a fluorescence-based single-molecule motility assay to quantitatively characterize motility parameters of single motors and clusters of Cin8. These experimental studies were complemented by predictive biophysical theory and computer simulations, that quantitatively agree with the experimental data. This interdisciplinary approach allowed us to quantify the response of Cin8 motors to external forces and interactions between Cin8 motors. On this basis, we have obtained a detailed understanding of the mechanisms underlying directional switching. The main insight is that directionality switching is caused by a single feature of Cin8: an asymmetric response of active motion to drag.
Importantly, our analysis shows that the very same mechanism can also explain multiple seemingly unrelated experimental factors which have been reported to induce switching of directionality of kinesin motors. Based on this mechanism, bidirectional motor proteins can switch directionality in response to clustering, increased ionic strength of the buffer, high motor density and molecular crowding, and in multi-motor motility assays.

